Vaccines are expensive and sensitive. They can lose their effectiveness if exposed to temperatures (heat and/or cold) outside the required range, and when exposed to light. Failure to adhere to storage requirements may reduce vaccine potency and/or increased local reactions after their administration. The loss of vaccine effectiveness is cumulative, permanent, and irreversible. Careful vaccine management is essential!
The Vaccines for Children (VFC) Program requires the Primary and Back-up VFC Coordinator to take the CDC You Call the Shots, Storage and Handling Training. It is also highly recommended that the Primary VFC Coordinator ensures that all staff handling vaccines have a good understanding of the vaccine ordering process, are familiar with the VFC Management Plan, and have completed You Call the Shots – Storage and Handling Training.
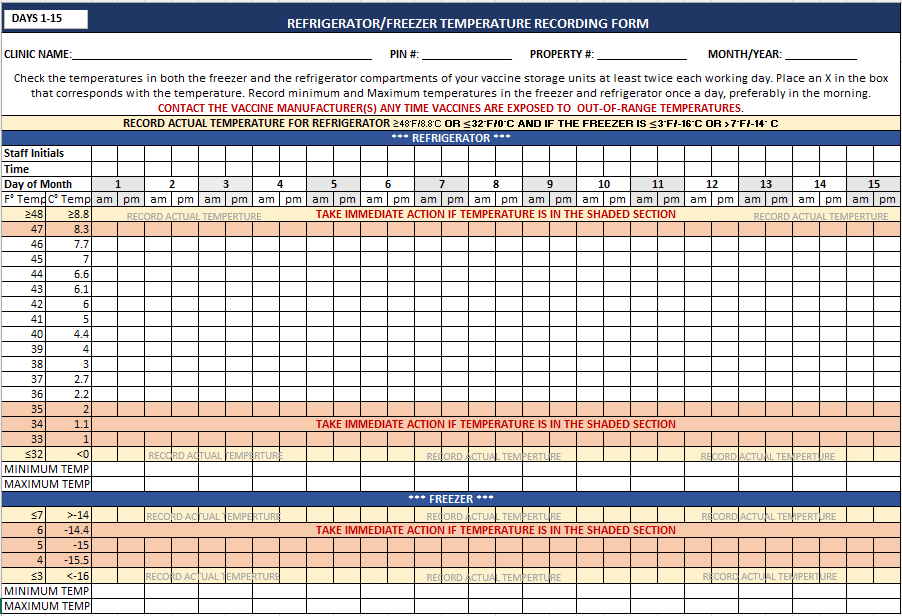
Providers are required to manually record the unit’s temperature twice a day to ensure that the VFC vaccines are maintained within a safe temperature range. The daily manual temperature recording may help expose early vaccine excursions and prevent vaccine loss.
Temperature documentation must include:
- The initials of the individual responsible for recording the temperatures.
- The time of the recording, and it must identify if it was an AM or PM recording.
- The current temperature must be recorded.
- The min/max temperature (if applicable) of the storage unit will need to be recorded each morning.
The Temperature Recording Log form should be placed on or near the storage unit(s) and must be made readily available to the VFC Representatives upon request. Temperature Recording Logs must be retained for three years prior to discarding. The same rule applies if the provider is no longer interested in participating in the VFC program.

Success is dependent on four key factors
- Well-trained staff
- The Immunization Branch encourages all VFC providers to fully train staff on routine and emergency vaccine management policies and procedures related to vaccine shipment, handling, transport, and inventory management to prevent vaccine loss.
- Reliable equipment
- Organize the refrigerator and freezer to facilitate vaccine management and reduce administration errors. Do not store vaccines until storage units have stabilized within the acceptable range for 3-5 days.
- Accurately completing the Temperature Recording log
- Manually record the unit’s temperature twice a day to ensure that the VFC vaccines are maintained within a safe temperature range.
- The initials of the individual responsible for recording the temperatures.
- The time of the recording, and it must identify if it was an AM or PM recording.
- The current temperature must be recorded.
- The min/max temperature (if applicable) of the storage unit will need to be recorded.
- Identify appropriate action to during a Temperature Excursion
- Respond to temperature excursions promptly.
- Vaccines exposed to temperature excursion should be labeled “DO NOT USE” until the vaccine manufacturer(s) are contacted regarding the vaccine viability.
- Document all the temperature excursions and actions taken.
- All VFC documentation should be retained for three years.


